Chlorophyll fluorescence technology-plant stress high temperature stress measurement technology
Chlorophyll fluorescence technology - plant stress high temperature stress measurement technology
With global warming, plant high temperature stress research has received more and more attention, and research methods are becoming more and more abundant, including plant fluorescence measurement: NPQ, Fv/Fm, OJIP, and Quantum Photosynthetic Yield . This article will focus on how to measure these fluorescence parameters efficiently, quickly and easily.
Non-photochemical quenching (NPQ) measurement
Non-photochemical quenching ( NPQ ) measurements are a good indicator of high temperature stress in plants (Schreiber U. 2004) (Tang Y., Wen X., Lu Q., Yang Z., Cheng Z., & Lu C. 2007) Haldiman P, & Feller U. 2004) , but the measurement process takes a long time and is very patient.
Typically, dark adaptations of 8-12 h or overnight are performed before field non-photochemical quenching ( NPQ ) is measured in the field . In the laboratory, dark adaptation time requires 12-24 h (Maxwell and Johnson 2000) . In addition to the factors such as the length of dark adaptation time and the success of dark adaptation, the measurement of NPQ needs to pay attention to the fact that it is necessary to start measuring after the blade reaches the photosynthesis steady state, which is easy to achieve, as long as the blade is placed in a stable state. The light level can be achieved in 15-20 min , usually using artificial light sources (Maxwell and Johnson 2000) .
Plant non-photochemical quenching can recover from the photo-suppressed state after a relaxation process of up to 60 h, so even if it is not enough overnight, there will always be some unfinished measurements in the NPQ process. It is judged that the size of the NPQ measurement is the Fm value in Fv/Fm (Baker 2008) , so it is important to select samples with the same or similar Fv/Fm when comparing the results .
The NPQ measurement is mainly used to study the high temperature stress at 35 °C and above. The NPQ saturated light flash curve can reflect the effect of high temperature stress on plants. The NPQ decreases with time, and the photosynthesis quantum yield increases with time. (
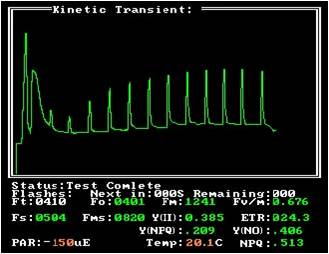
During the recovery period of high temperature stress, the sample was treated at 35 °C for 5 min and the recovery period at 20 °C.
Fv/Fm and OJIP measurements
 Fv/Fm and OJIP can be measured quickly, and the time to dark-adjust the sample before measurement can range from 20 min to overnight, and pre-dawn measurements can also be used. However, studies have shown that these two parameters have certain limitations for reflecting high temperature stress in plants. Studies have shown that Fv/Fm can only reflect high temperature stress around 45 °C , and OJIP can only reflect high temperature stress at 44 °C and above. (Haldiman P, &
Fv/Fm and OJIP can be measured quickly, and the time to dark-adjust the sample before measurement can range from 20 min to overnight, and pre-dawn measurements can also be used. However, studies have shown that these two parameters have certain limitations for reflecting high temperature stress in plants. Studies have shown that Fv/Fm can only reflect high temperature stress around 45 °C , and OJIP can only reflect high temperature stress at 44 °C and above. (Haldiman P, &
Quantum Photosynthetic Yield Measurement (â–³F/F')
The most suitable parameter for reflecting high temperature stress may also be the photosynthetic quantum yield ( â–³ F/F') .
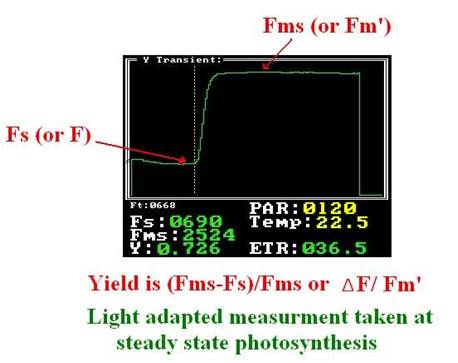 When the plant leaves reach the photosynthesis steady state under light conditions, or the plant leaves are placed under stable light level for 15-20 min, the â–³ F/F' parameter can be measured . (Maxwell and Johnson 2000).
When the plant leaves reach the photosynthesis steady state under light conditions, or the plant leaves are placed under stable light level for 15-20 min, the â–³ F/F' parameter can be measured . (Maxwell and Johnson 2000).
Numerous studies have shown that using natural light, the â–³ F/F' measurement is faster and faster, and only takes 2-5 s to complete. â–³ F/F' varies with the level of light, so either control the light level of the plant sample for comparison, or measure the actual light level of the leaf by PAR leaf clips and compare the measurements of similar light levels.
Cloudy weather can affect measurements when measured in the field. In addition, the blades that are directly illuminated by the sun are usually selected as the measurement object, because the spots and wind blows hinder the photosynthesis of the plant leaves to reach a steady state. 
The literature shows that â–³ F / F ' can reflect
△F/F' can be measured by many fluorescence measuring instruments, such as the US Opti-Sciences series instrument, OS5p multi-function modulation chlorophyll fluorescence instrument and OS1p portable modulation chlorophyll fluorescence instrument. NPQ is best used with the OS5p multi-function chlorophyll fluorescence instrument because it has a very stable activation source. The CO2 gas exchange measurement method can reflect high temperature stress around 30 °C (Haldiman P, & Feller U. 2004). For users who want to measure plant CO2 gas exchange and fluorescence parameters simultaneously, OS5P multi-function modulation chlorophyll fluorescence instrument and ADC can be used. LCpro+ plant CO2 gas exchange measurement combined system.
references
Baker NR, (2008) “Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo†Annu. Rev. Plant Biol. 2008. 59:89–113
Crafts-Brandner SJ, Law RD (2000) Effects of heat stress on the inhibition and recovery of ribulase-1, 5-biphsphate carboxylase/ oxygenase activation state. Planta (2000) 212: 67-74 Â
Dascaliuc A., Ralea t., Cuza P., (2007) Influence of heat shock on chlorophyll fluorescence of white oak ( Quercus pubescens Willd.) leaves. Photosynthetica 45 (3): 469-471, 2007
Haldimann P, &
Lichtenthaler HK, Burkart S., (1999) Photosynthesis and high light stress. Bulg. J. Plant Physiol., 1999, 25(3-4), 3-16
Lichtenthaler HK, Babani F. (2004) Light Adaption and Senescence of  From Photo 28, "Chlorophyll a Fluorescence a Signature of Photosynthesis", edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 716
Maxwell K., Johnson G. N, (2000) Chlorophyll fluorescence – a practical guide. Journal of Experimental Botany Vol. 51, No. 345, pp. 659-668- April 2000
Schreiber U, (2004) Pulse-Amplitude-Modulation (PAM) Fluorometry and Saturation Pulse Method: An Overview From Chapter 11, "Chlorophyll a Fluorescence a Signature of Photosynthesis", edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 279-319
Strasser RJ, Tsimilli-Michael M., and Srivastava A. (2004) - Analysis of Chlorophyll a Fluorescence Transient. From Chapter 12, Â "Chlorophyll a Fluorescence a Signature of Photosynthesis", edited by George Papaqeorgiou and Govindjee, published by Springer 2004, PO Box 17, 3300 AA Dordrecht, The Netherlands, page 340
Tang Y., Wen X., Lu Q., Yang Z., Cheng Z., Lu C., (2007) Heat stress induces an aggregation of the light-harvesting complex of photosystem II in spinach plants. Plant Physiology, Feb. 2007 Vol. 143, pp629-638
The urine bag set consists of A drainage bag and A suction device. Qhy-a is the single drainage bag, and qhy-b is the drainage bag + suction device. The drainage bag consists of the bag body, Catheter , lifting lug, joint, guard cap, drainage port, drainage bottom plug, anti-reflux device, and current limiting clip.The suction device is composed of negative pressure bottle, drainage tube joint, suction tube joint, suction connection pipe, automatic drainage device, drainage port and bag body.Should be sterile.
It is used for the collection of body fluids (blood, gastric juice, etc.), secretions (sputum, flushing fluid, etc.) and human excreta (urine, etc.) in hospital, clinical departments and during surgery or after surgery.
Urine Drainage Bag, Urinary Drainage Bags,Urine Collection Bags, dialysis drainage bag, drainage bag
Urometer Catheter Bag,Urine Meter Drainage Bag,Urine Night Bag,Reusable Urine Bag
Huali Technology Co., Ltd , https://www.jlhualitech.com