CFDA issued two registration review principles for artificial cervical disc prosthesis and hip prosthesis system
In order to strengthen the supervision and guidance of medical device product registration and further improve the quality of registration examination, the State Food and Drug Administration has formulated the Guidelines for the Technical Review of Artificial Cervical Disc Prosthesis Registration and the Technical Review Guidance for the Registration of Hip Prosthesis System. Principles (see annex), is hereby released.
announce.
Food and Drug Administration
February 10, 2017
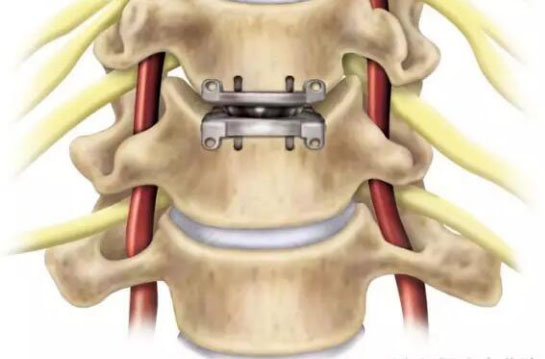
attachment1. Guidelines for the review of technical registration of artificial cervical disc prostheses
This guiding principle is intended to provide technical guidance for the applicant to register the artificial cervical intervertebral disc prosthesis, and also provide a technical reference for the review of the registration application materials by the food and drug supervision and administration department.
This guideline is a general requirement for the registration of artificial cervical intervertebral disc prosthesis. Applicants should enrich and refine the contents of the registration application according to the characteristics of the specific product, and determine whether the specific content is applicable according to the characteristics of the specific product. If not applicable, the reasons and corresponding scientific basis should be specified.
This guiding principle is a guiding document for applicants and reviewers, but does not include administrative matters involved in registration approval, and is not enforced as a regulation. If there are other methods that can meet the requirements of relevant laws and regulations, it can also be adopted, but it is required. Provide detailed research materials and verification materials. This guidance should be used in accordance with relevant regulations and standards.
This guiding principle is formulated under the current regulations and standards system and the current level of cognition. With the continuous improvement of laws and standards, and the continuous development of science and technology, the relevant content of this guiding principle will also be adjusted in a timely manner.
First, the scope of application
The products covered by this guideline are applicable to artificial cervical intervertebral disc prosthesis replacement, including upper endplate, nucleus pulposus prosthesis, lower endplate, made of metal and its alloys, coating materials, ultra high molecular weight polyethylene or ceramics. . This guideline does not apply to products such as elastic artificial nucleus, personalized customization, etc.
Second, the principle of division of registration units
The artificial cervical intervertebral disc prosthesis has a relatively simple clinical use, and each component is used for clinical use, and the coordination relationship between different sizes and specifications is relatively determined, so a single component is not declared as an independent unit. Components that are used as a single unit or combined can be declared in the same registration unit, such as: the same system component, the material is determined and unique, the upper end plate, the nucleus prosthesis, the lower end plate, although the material between the components Different, can be declared as the same registration unit. Different structural design types, such as motion retention type, motion limitation type, fixed structure type, and joint surface material combination, are declared as different registration units; similar components with different materials should be divided into different registration units.
Third, the registration application requirements
(1) Summary information
1. Management category, classification code and normative naming
According to the "Medical Device Classification Rules", "Medical Device Universal Name Naming Rules" and other related documents, according to the design characteristics and applicable scope of the declared products, determine their management categories, classification codes and normative naming, and discuss the basis for their determination.
2. Basic information about the product
mainly include:
(1) Product composition, number and name and number of each component; provide the division principle of each model specification of the product.
(2) The material grades of the components and coatings of the products and their conforming national standards, international standards, and industry standards, and the description of the material grades shall be consistent with the standards met. The description of the material grade of the imported product and the standards it meets shall be consistent with the certification documents/instructions of the country of origin, and not lower than the requirements of relevant national standards and industry standards.
(3) Surface modification treatment of the product, such as surface coating and its related preparation process; the surface of the product includes but is not limited to the upper and lower end plates and the vertebral body joint surface and the moving joint surface inside the product, and the coating includes but not Limited to bioactive coatings, wear-resistant modified coatings, etc., it is necessary to give information on the elemental composition, microstructure, physical and chemical properties, mechanical properties, bonding strength of the modified layer or coating and related preparation processes.
(4) Provide a structural diagram that reflects the structural characteristics and technical features of the product, as well as the geometric structure of each component and the functional description of each design feature; the typical structure of the product clearly defines the starting and ending points of the important dimensions and the overall description of the available size ranges; Product geometry, tolerances and surface roughness, such as product height, width, depth, arc of the end plate contact surface, inclination and other dimensions, as well as product rotation center height, joint surface diameter, etc. to identify product specifications, structure and compatibility The important dimensions and structure drawings should be based on the product CAD design drawings, and the design features of the products should be clarified from the overall appearance, the various dimension profiles, and the side and local details.
(5) Provide the scope of application, contraindications, and provide relevant scientific evidence to prove the rationality of its scope.
(2) Research data
1. Product performance study
For the products to be listed, the applicant should compare with the similar products on the market in terms of intended use, component materials, product structure, size range, static/dynamic mechanical properties and wear test to prove that they have the same safety and effectiveness.
(1) Experimental data on static and dynamic mechanical properties of artificial cervical intervertebral disc prosthesis and its components
In order to prove that the product can be safely and effectively applied to the intended patient in the long-term, the applicant should select the corresponding test item to verify according to the product condition, and select the worst case product for each test in the declared product to implement the corresponding mechanical performance test. The choice of the difference should indicate the reason, use the finite element analysis method if necessary and give an analysis report.
Lung Function Instrument Mouthpiece
Lung Function Instrument Mouthpiece,Plastic Disposable Mouthpiece,Medical Paper Mouthpieces,Disposable Paper Mouthpieces
Hengshui Qifei Paper Products Co. LTD , https://www.hengshuiqifei.com