Cell screening method and procedure [赛百慷]
Screening steps:
a. Determination of the killing curve 1. Spread the untransfected cells into a 24-well plate at 0.05 million per well and incubate overnight.
2. The next day, remove the old medium from the 24-well plate.
3. Add fresh medium containing different concentrations of puromycin (1ug/mL, 2ug/mL, 3ug/mL, 4ug/mL, 5 ug/mL, 6ug/mL, 7ug/mL) to the cell-coated 24 Orifice plate,
4. Replace the fresh screening medium every 2 days.
5. Observe the survival rate of the cells every day,
6. The minimum concentration of puromycin used is the lowest screening concentration that kills all cells within 1-4 days from the start of puromycin screening.
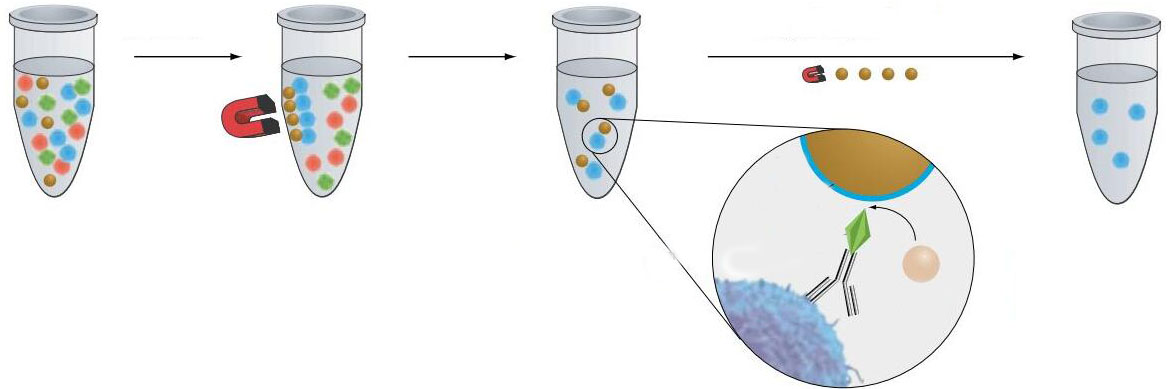
b. Puromycin selection of transfected cells 1. On the first day, the transfected cells were plated into a 24-well plate at 0.05 ml per well and incubated overnight. 2. The next day, the old medium in the 24-well plate was removed.
3. Add screening medium containing puromycin (2ug/mL), incubate,
4. Replace the fresh screening medium every 2 days.
5. Observe the survival rate of the cells every day,
6. Cells that survive at the same time point (2d) are successfully transfected cells.
7. Amplification of the screened cells.
Linaclotide,Teriparatide Acetate,Leuprorelin Acetate,Lypressin Acetate
PYSON Co. ,Ltd. , https://www.pysonbio.com