Summary of recent research progress in the field of cancer (03.26)
Summary of recent research progress in the field of cancer (03.26)
March 26, 2018 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];1. New drugs for prostate cancer are eligible for priority review
Recently, Pfizer and Astellas Pharma announced that the US FDA has accepted a new drug application for its innovative drug XTANDI (enzalutamide) and awarded it the priority for evaluation. If approved, XTANDI will be used for the treatment of non-metastatic castration-resistant prostate cancer (CRPC).

Prostate cancer is the second most common cancer in men worldwide. It is estimated that there will be 164,000 new-onset prostate cancer patients in the United States in 2018; in the European Union, this figure was 365,000 in 2015. Prostate cancer can be classified into several types, and prostate cancer, which still progresses when testosterone levels are close to castration, is called "castration-resistant prostate cancer." In such patients, cancer has not spread to other areas of the body, but prostate specific antigen (PSA) levels have increased. These patients are often difficult to escape the fate of cancer metastasis, so they need an effective drug to control their condition.
XTANDI is an androgen receptor inhibitor that has been approved for the treatment of metastatic castration-resistant prostate cancer. The new drug application submitted this time attempts to make this new drug treat patients before cancer metastasis. The FDA decision was based on a clinical trial called PROSPER, in which the efficacy of XTANDI in patients with non-metastatic castration-resistant prostate cancer was confirmed. The study enrolled a total of 1,401 patients, partially receiving androgen deprivation therapy (ADT), and the other receiving additional XTANDI. The results of the trial showed that the combination therapy of XTANDI and ADT significantly prolonged the patient's metastasis-free survival (MFS) compared with ADT. The median MFS of combination therapy was 36.6 months, while the median MFS of ADT monotherapy was 14.7. Months (n=1401, p<0.0001).
“Once cancer begins to spread and metastasize, patients with castration-resistant prostate cancer face a very poor prognosis, and survival rates are not optimistic,†said Dr. Steven Benner, Senior Vice President and Head of Global Therapy at Onsetia Cancer Development Says: "We are pleased to see the FDA's priority review and expect to bring this new drug to patients with non-metastatic castration-resistant prostate cancer."
"For patients with non-metastatic castration-resistant prostate cancer, treatment options are often limited. The only evidence that predicts this invasive disease is the rapidly rising PSA," Mace, Pfizer's global product development lead and chief oncology development officer. Dr. Rothenberg said: "XTANDI is already the standard treatment for metastatic castration-resistant prostate cancer. This milestone represents an important step in bringing XTANDI to early-stage patients."
2. Treatment of lung cancer, Tecentriq plus chemotherapy is expected to become a first-line therapy
Recently, Roche Group member Genentech announced the joint primary end point of its phase III clinical trial IMpower131 to progression-free survival (PFS), demonstrating that Tecentriq (atezolizumab) is combined with chemotherapy (carboplatinum and ABRAXANE®). First-line treatment of patients with advanced squamous non-small cell lung cancer (NSCLC) can reduce the risk of disease progression or death, and the effect is better than chemotherapy alone.

According to the American Cancer Society, more than 234,000 Americans will be diagnosed with lung cancer in 2018, with non-small cell lung cancer accounting for 85%. It is estimated that about 60% of lung cancers in the United States are diagnosed at an advanced stage. The prognosis of these patients is not optimistic, and new and effective treatments are urgently needed to alleviate the disease and prolong life.
Tecentriq is a heavyweight immunization drug for Genentech and is currently approved for treatment of urothelial carcinoma and NSCLC in the United States. It is an anti-PD-L1 monoclonal antibody that binds to PD-L1 on tumor cells and tumor-infiltrating immune cells, blocks its interaction with PD-1 and B7.1 receptors, and reactivates T cells. Kill cancer cells.

The IMpower131 was a phase 3 open-label, multicenter, randomized study evaluating Tecentriq binding to carboplatin and albumin-bound paclitaxel (nab-titosterol) or Tecentriq in combination with carboplatin and paclitaxel and chemotherapy alone The efficacy and safety of patients with stage IV squamous NSCLC who had not received chemotherapy before (carboplatin and nab-paclitaxel). The study enrolled 1021 patients randomized to receive a 1 : 1 ratio of 1) Tecentriq plus carboplatin and paclitaxel (group A); 2) Tecentriq plus carboplatin and nab-paclitaxel (group B); 3) Carboplatin and nab-paclitaxel (group C, control group). The primary primary endpoints of the study were 1) PFS as determined by the investigator in the intention-to-treat (ITT) population by RECIST v1.1 (Group B vs. Group C); and 2) Total survival determined in the ITT population (OS) ) (Group B vs. Group C). The results showed that the study reached the common primary endpoint of its PFS. In addition, the safety of Tecentriq in combination with chemotherapy is consistent with the safety of known individual drugs, and no new safety signals have emerged.
“Squamous non-small cell lung cancer is difficult to treat, and there have been few new treatment options in the past few decades,†said Dr. Sandra Horning, Chief Medical Officer and Head of Global Product Development at Genentech. “We will share with global health regulators. The results of IMpower131 and look forward to seeing more mature overall lifetime data."
3. New breakthroughs in immunotherapy! Enriched tumor infiltrating lymphocytes have excellent anticancer effect
One of the immunotherapy that has achieved many results in the cancer treatment field in recent years is genetically engineered T cell therapy, which extracts immune cells from the patient's own blood, and modifies the T cells with anticancer effects so that they can recognize and Killing cancer cells, in vitro expansion and then returning to patients, in order to achieve the purpose of specifically killing cancer cells.
This therapy has been successful in blood cancers such as leukemia and lymphoma, but the effects on solid tumors are still not satisfactory. The researchers believe that the reason for this is that the mutation of the solid tumor is not enough to activate the body's immune system to kill it. If T cells can be extracted directly from tumor tissues, and then returned to the patient after in vitro culture, it may bring better results.

Recently, researchers from the Ludwig Institute for Cancer Research in Lausanne, Switzerland, found that T cells extracted from tumors have better anticancer effects than T cells extracted from blood. Published in Nature Communications.
There are T cells in epithelial ovarian tumors that are particularly good at identifying and killing cancer cells. Extracting these tumor infiltrating lymphocytes (TILs) and amplifying them and returning them to patients is not a new idea, but previous attempts have not been successful. According to Ludwig's scientist Dr. Alexandre Harari, when these cells grow in an in vitro culture environment, the proportion of cells that recognize cancer cell mutations often decreases. Therefore, the Ludwig team developed a new technology to identify those TILs that are "highly reactive" to achieve the effect of amplifying them in vitro rather than diluting them. Then, the researchers compared the activity of TIL extracted from tumors and T cells extracted from blood in targeting neoepitope, which are all fragments of antigenic mutations that remind the body. The immune system has cancer.

The researchers found that engineered killer TILs from ovarian tumors are better at locating newborn antigenic determinants than blood-derived T cells. Moreover, they found "highly reactive" killer T cells in approximately 90% of ovarian cancer patients, suggesting that such therapies have potential for widespread use. The next step for the Ludwig team will be to apply this discovery to other projects being carried out by the center to develop personalized treatments for cancer patients.
"The most important message from our findings is that in the future, when designing engineered cell-based therapies, tumor-specific TILs should be prioritized for low-mutation-loaded tumors, and their anticancer effects will be superior to those collected from peripheral blood. T cells," said Dr. George Coukos, director of the Ludwig Cancer Institute, "This new strategy to enrich TILs also provides patients with good treatment opportunities."
4. New T cell therapy is expected to treat a variety of solid tumors
Adaptimmune Therapeutics, a biopharmaceutical company that develops new T-cell therapies, has announced that NY-ESO SPEAR T cell therapy has partially relieved patients with the first mucin-like round cell liposarcoma (MRCLS). This is also the second solid tumor treated by this new T cell therapy. This new platform technology is expected to achieve efficacy in a variety of solid tumors.

Both MRCLS and synovial sarcoma are soft tissue sarcomas. MRCLS is a liposarcoma characterized by proliferation of adipocyte precursors and stagnation of differentiation. This malignant tumor is derived from the translocation of chromosomes 12 and 16, resulting in the fusion protein blocking adipocyte differentiation and promoting malignant transformation. Synovial sarcoma is characterized by a translocation of the X chromosome and chromosome 18, which is different from the origin of the immature adipocytes known by MRCLS, and the origin cells of synovial sarcoma are still unknown. It is estimated that there are approximately 2,000 MRCLS patients in the United States and Europe each year. MRCLS has the highest incidence among patients aged 30 to 50 years and is usually more aggressive than other liposarcomas. MRCLS also exhibits a unique metastatic pattern that first appears in the proximal extremities of the limbs, usually spreading to the bones (especially the spine), the serosal surface, the retroperitoneum, the abdomen, the pelvis, and other soft tissues. This mode of metastasis is different from the lung diffusion profile exhibited by synovial sarcoma.
Adaptimmune's new cancer immunotherapy SPEAR (Specific Peptide Enhanced Affinity Receptor) T cell platform enables T cells to engineer and target cancer, including solid tumors. SPEAR T cells targeting MAGE-A4, -A10 and AFP are undergoing clinical trials in a variety of solid tumor indications. The results reported recently showed that three of the top four patients had partial remission (two confirmed and one to be confirmed), and one patient was stable. Patients are well tolerated by cytokine release syndrome (CRS) according to standard treatment guidelines.
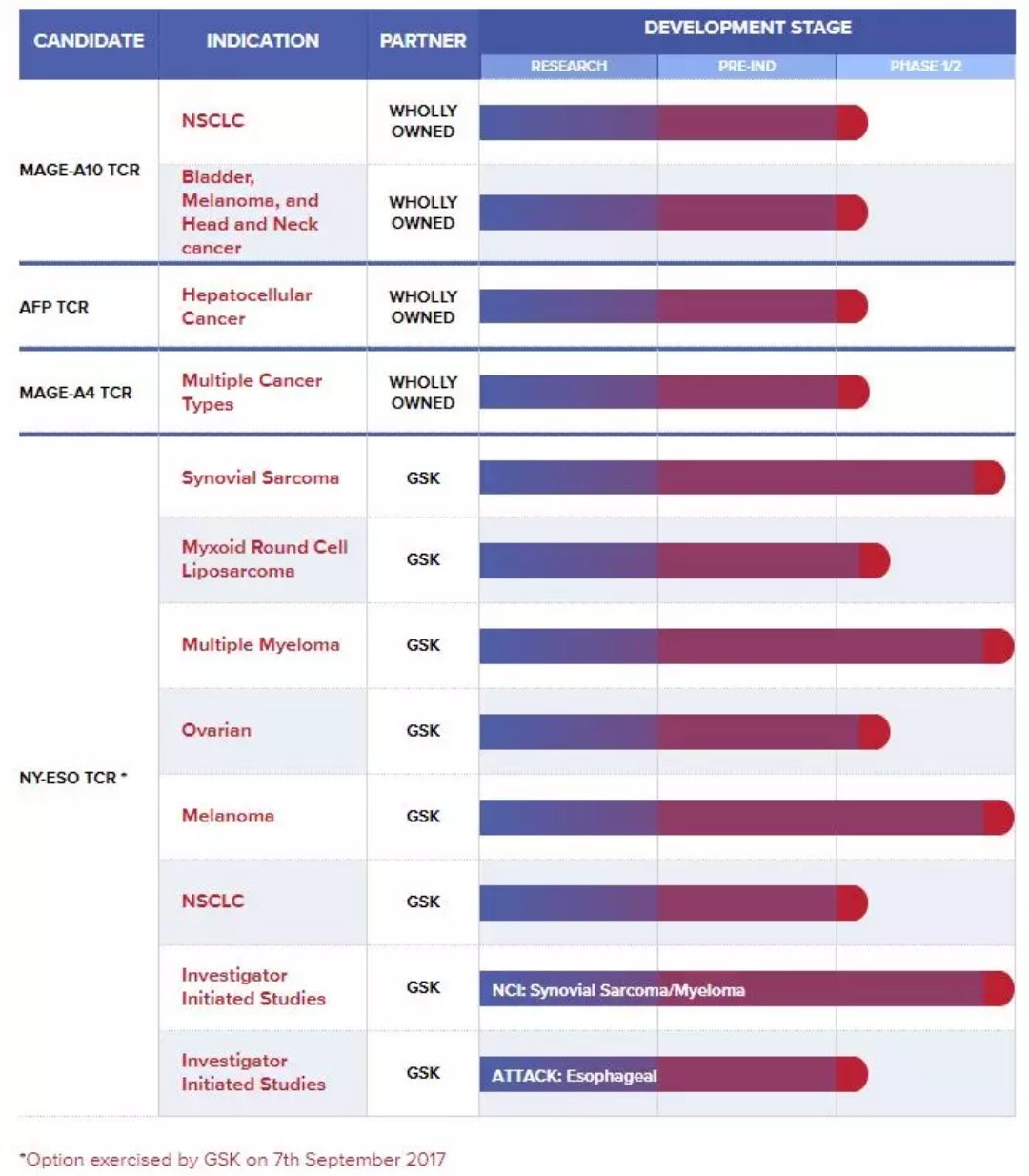
â–²Adaptimmune Therapeutics research and development pipeline (Source:Adaptimmune Therapeutics official website)
"We are encouraged by the initial remission of MRCLS patients who have used NY-ESO SPEAR T cell therapy because it validates the potential of our platform to treat a wide range of tumors, including those that are not alleviated by existing immunotherapies," Adaptimmune Chief Medical Dr. Rafael Amado said: "Although MRCLS is a soft tissue sarcoma that normally expresses NY-ESO, its clinical course, natural history, molecular markers and reactivity to standard treatment are fundamentally different from synovial sarcoma. Together with more data from other trials, these results on the second solid tumor enhance our belief that our unique TCR pipeline will be able to solve a variety of solid tumors."
Reference material
[1] FDA Grants Priority Review for Prostrate Cancer Treatment
[2] Genentech's Tecentriq Shows Promise for First-line Lung Cancer Treatment
[3] Tumor-derived T-cells show promise against ovarian cancer
[4] Adaptimmune shares boosted by new signs of success with TCR cell therapy
Original title: Summary of recent research progress in the field of cancer (No. 59)
Concealed Hinges,Screw-On Hinges,Concealed Door Hinges,Concealed Cabinet Hinges
Ningbo Hengchieh Locking Technology Co., Ltd. , https://www.yh-lock.com