Shanghai Lei magnetic automatic titrator for the determination of monosodium glutamate, total acid in chicken essence and sodium glutamate
First, the application background
MSG, chicken essence to increase the flavor of food, the main ingredient is sodium glutamate. Sodium glutamate is a sodium salt of amino acid glutamic acid. It is a colorless, odorless crystal that melts and disintegrates at 232 °C. Eating MSG or chicken essence will not cause any damage to health in the normal range, but excessive consumption may cause headache, redness, sweating, facial compression or swelling, numbness in the mouth or mouth, burning sensation in the stomach. Symptoms of poisoning such as chest pain.
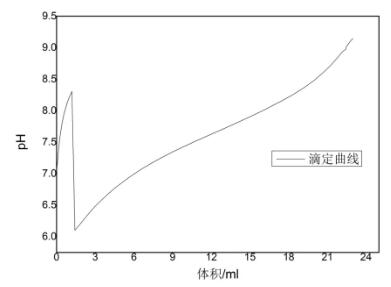
We use potentiometric titration to determine the content of total acid and sodium glutamate in monosodium glutamate and chicken essence. This method can be used to determine total acid and sodium glutamate by one titration. It is simple, fast, convenient and easy to operate.
Second, according to the standard
SB/T10371-2003 Chicken Condiment
NY/T1053-2006 Green Food MSG
GB2720-2003 MSG Hygiene Standard
Third, recommended instruments and reagents
1. Instrument: Thundermagnetic ZDJ series titrator (potentiometric titration)
Electrode: 231-01pH glass electrode, 232-01 calomel reference electrode
2. Reagent: 0.05mol/L sodium hydroxide standard titration solution (preparation and calibration reference file M9103-2015) formaldehyde
Fourth, sample determination
1. Shake MSG or chicken essence, accurately weigh 0.50g sample into the titration cup, add 50mL pure water. At the same time, do a blank test.
2. Instrument operation:
a) Turn on the instrument, connect the 231-01 pH glass electrode to the measuring electrode interface, and connect the 232-01 calomel reference electrode to the reference electrode interface.
b) Calibration electrode: Select “mV/pH†button, click recalibration, put the electrode into 4.00 pH standard buffer solution, wait for the value to stabilize, press “calibrationâ€, clean the electrode and put it into 9.18 pH standard buffer solution. After the value is stable, press “Calibration†to end the calibration.
c) Place the electrode holder above the titration cell and place the measuring electrode, reference electrode and filling tube.
d) Connect the liquid take-up tube to the sodium hydroxide standard titration solution, use the instrument to clean the button, clean it three times, and connect the cleaning solution to the waste cup.
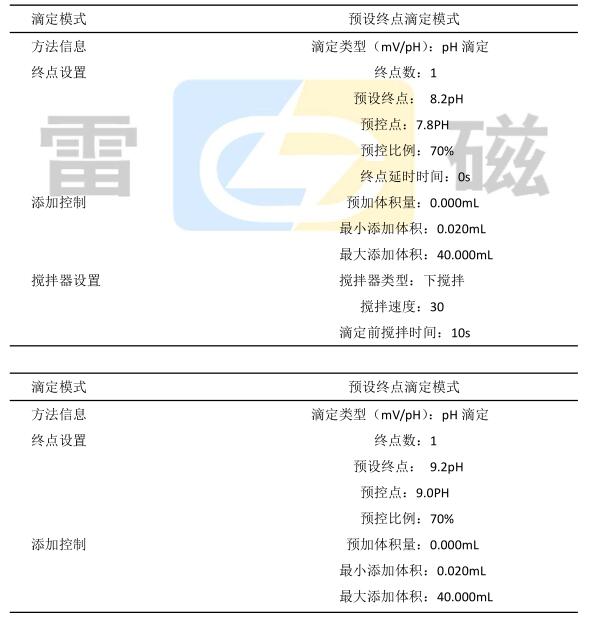
e) Click Start Method Titration, select the preset end point titration, and set the parameters.
f) Click to start the measurement and wait for the instrument to automatically detect.
g) At the end of the analysis, the instrument displays the conclusion and clicks Save.
h) After the determination of total acid, manually add 10mL of formaldehyde solution, click the start method to titrate, select the preset end point titration, set the parameters, and determine the amino nitrogen content.
i) At the end of the analysis, the instrument displays the conclusion and clicks Save.
j) If it is not measured, use the cleaning button, connect the infusion tube to pure water and wash it three times.
k) Turn off the instrument and soak the electrode in saturated potassium chloride.

Fifth, the calculation formula
The total acid content of the sample (calculated as lactic acid) is calculated as follows:
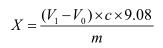
among them:
X—the total acid content (in lactic acid) in the sample, in grams per hundred grams (g/100g);
V1—the value of the volume of the sodium hydroxide standard titration solution consumed by the sample for measurement, in milliliters (mL);
V0—the value of the volume of the sodium hydroxide standard titration solution consumed by the reagent blank, in milliliters (mL);
M—the value of the mass of the sample, in grams (g);
c—The actual concentration of the sodium hydroxide standard titration solution, in moles per liter (mol/L).
The content of amino nitrogen in the sample is calculated by the following formula:
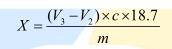
among them:
X—the content of amino nitrogen in the sample, in grams per hundred grams (g/100g);
V3—the value of the volume of the sodium hydroxide standard titration solution after the addition of formaldehyde to the sample for measurement, in milliliters (mL);
V2—Reagent blank test The value of the volume of sodium hydroxide standard titration solution after adding formaldehyde, in milliliters
(mL);
M—the value of the mass of the sample, in grams (g);
c—The actual concentration of the sodium hydroxide standard titration solution, in moles per liter (mol/L).
Six, matters needing attention
1. After removing the electrode sheath, the sensitive glass bulb of the electrode should be avoided from contact with the hard object, because any damage or rubbing will invalidate the electrode.
2. At the end of the measurement, the electrode protection sleeve should be placed in time. A small amount of external reference solution should be placed in the electrode sleeve to keep the electrode bulb moist. Do not soak in distilled water.
3. The electrode should be immersed in distilled water, protein solution and acidic fluoride solution for a long time.
4. The filling liquid of the reference electrode is 3mol/L potassium chloride solution. The filling liquid can be added from the small hole at the upper end of the electrode. When not used for a long time, the liquid filling hole is plugged and a protective sleeve is installed to prevent the filling liquid from drying up.
(Content source Lei magnetic official website)
Time Attendance,Time Attendance Software,Face Recognition Attendance,Card Recognition Time Attendance
Chongqing Huifan Technology Co., Ltd , https://www.huifantech.com