Science Supplement: The new role of β5i in the development of cardiac hypertrophy
Pathological cardiac hypertrophy can lead to heart failure if not adequately treated. A recent report by a group of researchers indicates that in angiotensin II (Ang II)-treated cardiomyocytes and hypertrophic hearts, the expression and activity of the immunoproteasome catalytic subunit β5i is significantly increased, and this effect is in cardiomyocytes and Overexpression of β5i is exacerbated in transgenic mice. This suggests a novel role for β5i in the regulation of cardiac hypertrophy, so inhibition of β5i activity may serve as a novel treatment for cardiac hypertrophy.
The research results were published in the May 8 issue of Science Advances. The author of the article was Professor Li Huihua, Dean of the School of Public Health, Dalian Medical University, and Wang Hongxia, Capital Medical University. Professor Li for cardiovascular disease (including heart failure, myocardial infarction) The pathophysiological mechanisms of the development of hypertension, arrhythmia, etc., mainly explore the pathophysiological mechanisms of ubiquitin protein modification, immune inflammation and imbalance of nutrient metabolism to regulate cardiovascular diseases.
Heart failure is the final stage of various heart diseases and is a major cause of morbidity and mortality worldwide. Two features of chronic heart failure are cardiac hypertrophy and decreased myocardial contractility. As a precursor to heart failure, cardiac hypertrophy is a very rigid response of the heart to long-term stresses such as chronic stress, volume overload or neurohormonal stimulation.
Cardiac hypertrophy can generally be divided into physiological or pathological hypertrophy. The initial hypertrophic response can be used to maintain cardiac output. After the initial phase of supplementation, if hypertrophic growth leads to cardiac dysfunction, it is considered pathological hypertrophy.
To date, a variety of signaling pathways have been shown to positively regulate protein synthesis and cardiac hypertrophy, including insulin-like growth factor 1 receptor (IGF1R)/phosphatidylinositol 3-kinase (PI3K)/AKT, epidermal growth factor receptor (EGFR). / mitogen-activated protein kinase (MAPK), gp130 / Janus kinase (Jak) / signal transduction and transcription activator 3 (STAT3), and calcineurin / nuclear factor of the activated T cell (NFAT) pathway. Therefore, therapeutic strategies for the expression and activation of these receptors and downstream media are very promising.
The ubiquitin-proteasome system (UPS) and the lysosomal-autophagy pathway are the two most important cellular mechanisms of protein degradation. Autophagy is generally considered to be a non-specific process that degrades long-lived proteins and abnormal protein aggregates. In contrast to autophagy, UPS is the primary pathway for intracellular protein degradation in eukaryotic cells. The standard 20S proteasome contains three beta subunits, β1 (PSMB6), β2 (PSMB7) and β5 (PSMB5), which represent the caspase-like, trypsin-like and chymotrypsin-like activities of the proteasome, respectively. .
After stimulation with cytokines such as interferon-gamma (IFN-γ), three alternative beta subunits (also known as immunological subunits): β1i [large multifunctional peptidase 2 (LMP2) or PSMB9)], β2i (MECL- 1 or PSMB10) and β5i (LMP7 or PSMB8) were induced to replace their standard subunits to form a 20S immunoproteasome.
The primary function of the immunoproteasome is to improve primary histocompatibility complex-I (MHC-I) antigen presentation. The immunoproteasome also has a variety of biological functions including pro-inflammatory cytokine production, T cell differentiation and survival, oxidative stress and muscle mass.
Previous studies by Professor Li showed that the expression of the immune subunit β5i and chymotrypsin-like activity in the heart increased significantly under hypertrophic stress. Moreover, their data show that β5i is also involved in the regulation of avascular angiotensin II (Ang II)-induced atrial fibrillation. However, the function of β5i in regulating cardiac hypertrophy remains unknown.
In this study, members of the study group analyzed primary cardiomyocytes, β5i knockout (KO) mice, and cardiac-specific β5i transgenic (β5i-Tg) mice (provided by Simei Biotech to provide transgenic mouse services) . A new role for β5i in the development of cardiac hypertrophy was discovered—as a novel positive regulator of cardiac hypertrophy induced by Ang II, pointing out the necessary compensatory link between immunoproteasome and autophagy in hypertrophic hearts.
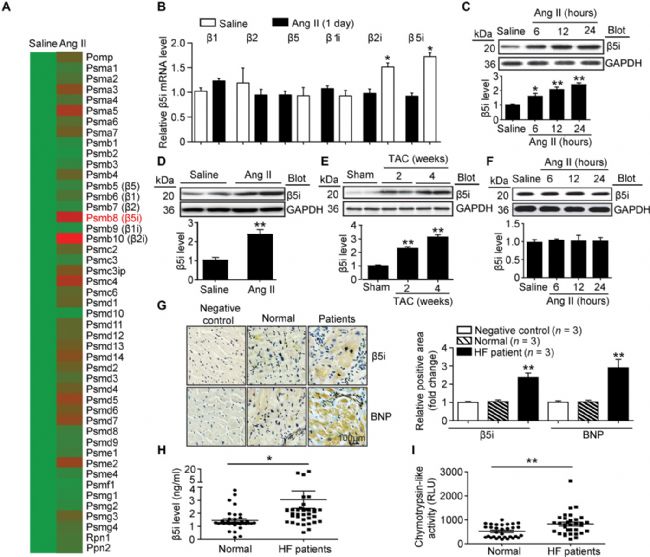
Increased expression of β5i in hypertrophic heart and HF patientsTo identify proteasome subunit expression profiles in damaged myocardium, the researchers induced acute cardiac hypertrophy in wild-type (WT) mice by Ang II infusion and analyzed proteasome subunit expression using a microarray assay.
As a result, they found that among the 47 proteasome subunit genes, the immune subunit β5i (also known as PSMB8 or LMP7) was significantly up-regulated in the heart on the first day of Ang II infusion. The expression of β5 imRNA was then confirmed by quantitative polymerase chain reaction (qPCR) analysis.
At the same time, the mRNA level of β2i (also known as PSMB10) in the Ang II treated heart increased, but was less than the mRNA level of β5i. However, there was no difference in the expression of standard subunits (β1, β2 and β5) and immune subunit β1i in cardiac tissue after Ang II treatment. In addition, β5i protein levels were increased in Ang II treated neonatal rat cardiomyocytes (NRCM). After 2 weeks of infusion of Ang II, the expression of 5i was also increased in the mouse heart. There was no change in β5i expression in neonatal rat cardiac fibroblasts following Ang II stimulation (Fig. 1).
(figure 1)
To examine whether β5i has the same important role in human HF, the researchers analyzed the expression of β5i and B-type natriuretic peptide (BNP; HF marker) in cardiac tissue. Immunohistochemistry showed that the expression of β5i and BNP in the failing heart was significantly higher than that in the normal control group.
In addition, serum β5i and chymotrypsin-like activity levels were also increased in HF patients compared to the control group. The researchers then analyzed the relationship between serum β5i or chymotrypsin-like activity levels in patients with HF by simple cross-tabulation and odds ratio (ORs) calculations. Multivariate logistic regression analysis examined the independent contribution of serum β5i or chymotrypsin-like activity levels to HF. These results indicate that β5i may play a key role in the regulation of cardiac hypertrophy.
Knock out β5i to inhibit cardiomyocyte hypertrophy in vitro
To determine the effect of β5i on function in cardiac hypertrophy, the researchers first examined whether β5i exerts a pro-hypertrophy or hypertrophic effect in vitro. They found that knockdown of β5i attenuated Ang II-induced increases in cardiomyocyte size and atrial natriuretic factor (ANF) and BNP expression compared to siRNA controls.
To confirm these findings, the researchers conducted further studies and found that Ad-β5i infection of NRCM increased the β5i level by 2.3-fold, increased myocardial cell size, ANF and compared with the Ad-GFP control group after Ang-II treatment. Increased BNP expression. Furthermore, siRNA knockdown of β5i reduced the phosphorylation levels of AKT and extracellular signal-regulated kinase 1/2 (ERK1/2) compared to the siRNA control after Ang2 stimulation. However, knockdown or overexpression of β5i has no effect on cardiomyocytes under basal conditions.
Next, they analyzed the effect of β5i on phenylephrine-treated cardiomyocytes. Consistent with the data from Ang II treated cells, knockdown of β5i also reduced phenylephrine-induced cardiomyocyte hypertrophy and ANF expression compared to siRNA controls. These data indicate that knockdown of β5i inhibits cardiomyocyte hypertrophy in vitro.
55i regulates cardiomyocyte size by autophagy
To determine whether β5i regulates cardiomyocyte size by autophagy, the researchers infected erythrocytes with siRNAs, reducing levels of endogenous β5i and ATG5. Western blot analysis showed that siRNA-β5i and siRNA-ATG5 achieved a decrease in β5i and ATG5 levels, respectively, compared to the siRNA-control.
Furthermore, knockdown of β5i attenuated Ang II-induced cardiomyocyte size, ANF expression, and increased phosphorylation of AKT and ERK1/2 compared to siRNA controls, which reduction was completely reversed by knockdown of ATG5 or β5i and ATG5.
To further confirm the effect of autophagy on cardiomyocyte size and related signaling pathways, the researchers infected cardiomyocytes with siRNA-β5i and treated with chloroquine, an autophagy inhibitor. They found that knockdown of β5i increased ATG5 expression compared to siRNA controls, and chloroquine treatment further enhanced this effect. Consistent with the observation of siRNA-ATG5-infected cells, β5i knockdown-mediated Ang II-induced increase in cardiomyocyte size, ANF expression and attenuation of AKT and ERK1/2 phosphorylation were also treated by chloroquine in siRNA-β5i-infected cells. reverse. In addition, the inhibition of cardiomyocyte hypertrophy by β5i knockdown can be restored only by chloroquine.
Taken together, these results indicate that blocking β5i activity can be compensated by induction of autophagy, and inhibition of autophagy reverses the protective effect of β5i knockdown.
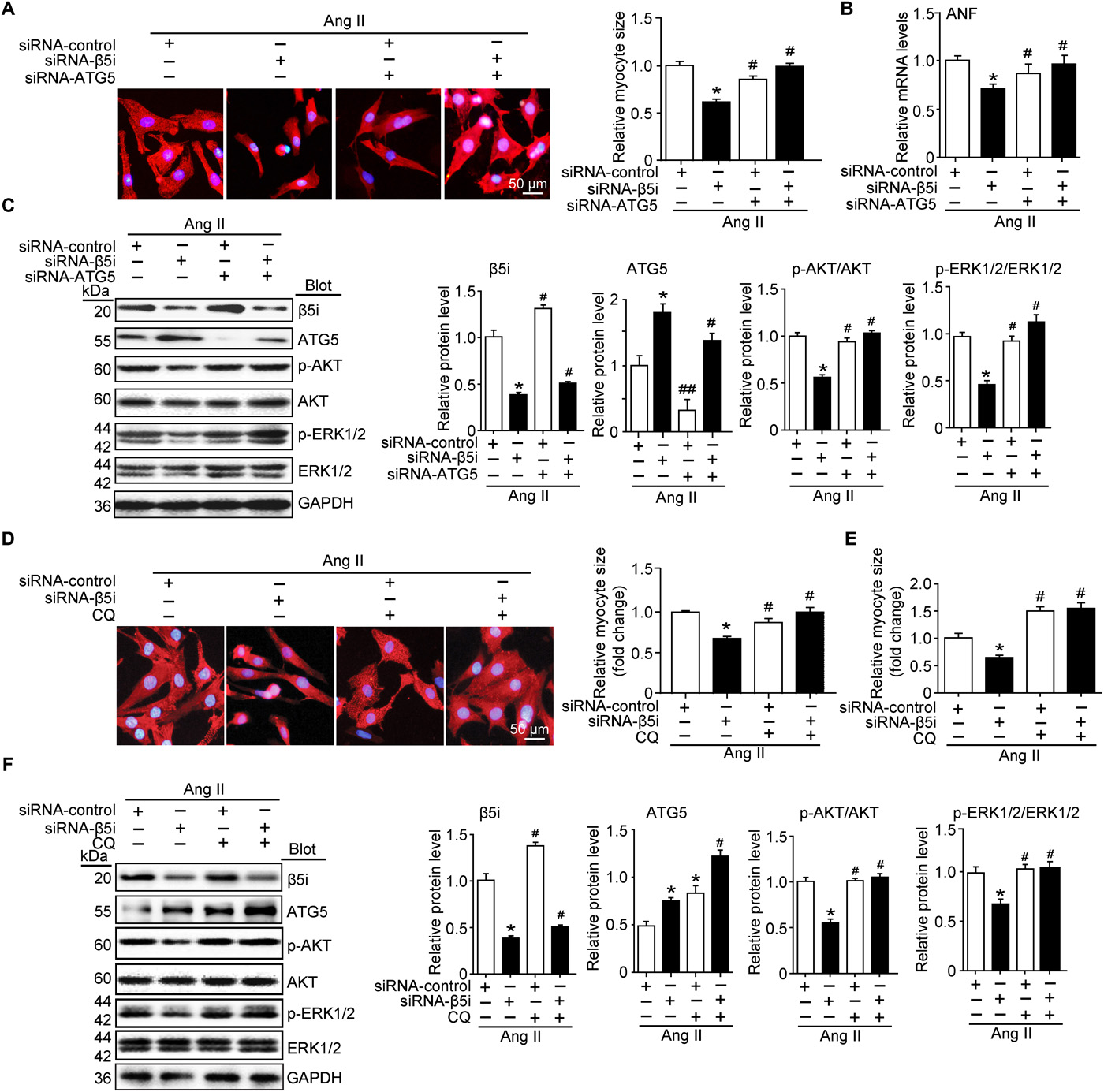
figure 2
Knocking out ATG5 can counteract the cardioprotective effects of β5i-deficient mice under pressure overload
To test whether ATG5-dependent autophagy mediates inhibition of cardiac hypertrophy by β5i deletion, the researchers injected WT or β5iKO mice with rAAV9-siRNA to knock down endogenous ATG5 expression. Three weeks after injection of rAAV9-siATG5, they found that AAV9-siATG5 injection significantly reduced ATG5 protein levels in the heart compared to rAAV9-sicontrol. Compared with rAAV9-sicontrol, β5iKO mice showed improvement in cardiac function.
Thus, knockdown of ATG5 in β5iKO mice also reduced the LC3-II/I ratio, but increased ERK1/2 activation, compared to rAAV9-sicontrol mice. ATG5 knockdown in WT mice has a similar effect compared to the RAAV9-sicontrol animals after TAC. Therefore, these in vivo data indicate that β5iKO attenuates cardiac hypertrophy by increasing the level of ATG5.
summary
In conclusion, this study found that the immune subunit β5i has a novel role in promoting key regulators of pathological hypertrophy. 55i targets ATG5 degradation and inhibits autophagy activation, leading to cardiac hypertrophy and dysfunction, indicating a necessary compensatory relationship between the immunoproteasome and autophagy activation in the cardiac hypertrophy program.
Next, the team will further determine which E3 ligase promotes ubiquitination of ATG5 and determine whether inhibition of β5i can be a new strategy for the treatment of hypertrophic diseases.
About the Author:
Li Huihua, Dean of School of Public Health, Dalian Medical University, Director of Cardiovascular Research Institute, Distinguished Professor of Education, Jiang Scholar, winner of the National Science Fund for Distinguished Young Scholars. He received his Ph.D. from Peking Union Medical University in 1998. He was a postdoctoral research assistant at the Department of Pathology at Wake Forest University and the Cardiovascular Biology Research Center at the University of North Carolina in 1998-2005. He was admitted to Peking Union Medical College from 2005 to 2010. Professor of the Department of Science, Professor of Capital Medical University from 2010 to 2014, and worked at Dalian Medical University from 2015 to the present. In recent years, he has been selected as one of the “National Talents Project†and has won the “Outstanding Young and Middle-aged Expertsâ€, the Chinese Overseas Chinese Innovation Talents Contribution Award, the State Council Special Allowance, and the Liaoning Provincial Climbing Scholar.
research direction:
Pathophysiological mechanisms of cardiovascular disease (including heart failure, myocardial infarction, hypertension, and arrhythmia). It mainly discusses the pathophysiological mechanism of ubiquitin protein modification, immune inflammation and nutrient metabolism imbalance in regulating cardiovascular diseases.
Original title:
The immunoproteasome catalytic β5i subunit regulates cardiac hypertrophy by targeting the autophagy protein ATG5 for degradation
Plant extracts can be used in animal husbandry materials. Traditional Chinese medicine extracts can improve animal immunity, survival rate and reproduction rate. Can protect the animal's gut flora from being destroyed,
Animal Husbandry Materials,Andrographis Extract,Amur Corktree Bark Extract,Cortex Phellodendri Extract Powder
Fufeng Sinuote Biotechnology Co.,Ltd. , https://www.sinuotebio.com