Electrotransformer efficiently transfects mRNA into mouse fertilized eggs to form stable mutants
With the completion of sequencing of mouse genome sequences, many studies have centered on the biological processes involved in functional genes, particularly in the field of developmental and disease research. So stable genetically modified model animals are important for elucidating the role of genes. However, traditional methods, including homologous recombination in embryonic stem cells or construction of mouse chimeras, are time consuming and labor intensive. However, the new genome editing method significantly optimizes this process. The CRISPR/Cas 9 system is the easiest way to construct a modified genome in an efficient genome editing approach.
Although the CRISPR/Cas9 system simplifies the process of forming mutants, the microinjection of DNAs/RNAs into fertilized eggs requires special techniques, and it takes a lot of time to obtain a large number of mutants (injecting hundreds of fertilized eggs). So, to simplify this process, we tried to introduce RNA into the fertilized egg using an electric converter.
Electrotransfers have also been used in mouse fertilized eggs, but the process involves removing the zona pellucida or thinning it with acid, which is very toxic to cells and has only short RNAs (less than 1 kb). Was imported.
Recently, a rat mutant was successfully constructed by transfecting Cas9 mRNA and gRNA into rat fertilized eggs by keeping the zona pellucida intact. However, genome editing is inefficient (less than 9%) and requires a large amount of Cas9 (1000-2000 ng/ul).

The right box is the electroporator CUY21 EDIT II (BEX Co.Ltd), which generates electric pulsed, and The left is a stereoscopic microscope for embryo manipulation. Two electrodes were placed on the microscope stage and connected to the electroporator.
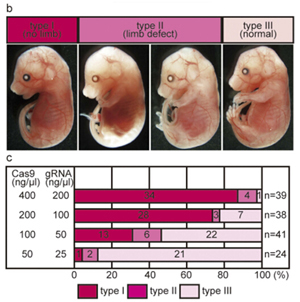
Figure 2. CRISPR/Cas9 mediated genome editing by electroporation. (b) Examples of embryos from the three classes that exhibit different limb phenotypes: no limbs (type I), limb defects (type II, left hind limb deficiency, right: truncated fore- And hind-limb) , and normal (type III). (c)Graph summarizing the effects of Cas9 and gRNA electro-poration on limb development. The concentrations of RNAs used in each experiment are shown at left. The numbers in each column are The number of embryos exhibiting the phe-notype in each category.
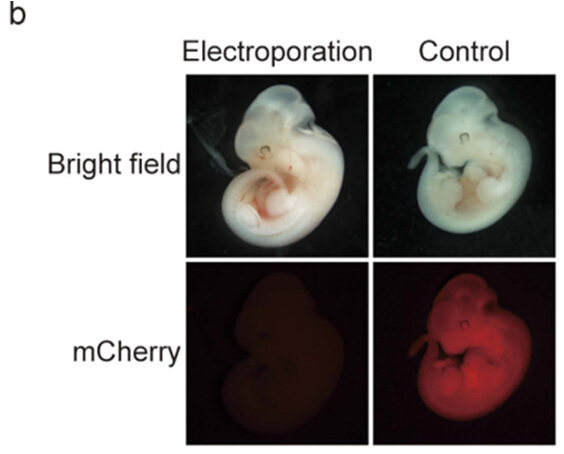
Figure 3. The single-stranded oligodeoxynucleotide (ssODN)-induced generation of HDR-mediated instertions. (b) Representative embryo electroporated with Cas9 mRNA, gRNA, and ssODN.The mCherry florescene completely disappeared in the electroporated embryos, while control (no electroporation Embryos displayed florescent signals.
Next, we reveal whether this mutation will be inherited in the next generation. Electroporation 200ng / ul Cas9 mRNA and 100ng / ul of gRNA into the egg cell, gene targeting mCherry. Twenty- five F0 mice were observed , and no red fluorescence was expressed. Moreover, all F1 mice did not have red fluorescence and the mCherry gene was interrupted. This indicates that the gene editing mutant can be stably inherited.
discuss

Best Water Distiller,Distilled Water Machine,Podiatry Water Distiller,Portable Water Distiller
ZHEJIANG FOMOS MEDICAL TECHNOLOGY CO.,LTD. , https://www.ifomos.com